Comprehensive Organic Name Reactions and Reagents, 3 Volume Set
Versandkostenfrei!
Versandfertig in über 4 Wochen
744,99 €
inkl. MwSt.

PAYBACK Punkte
372 °P sammeln!
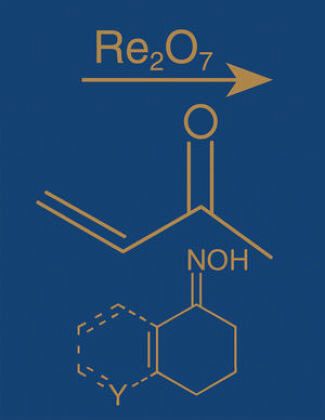
Jeder Eintrag enthält eine Beschreibung der Reaktion, ein Diagramm zum Reaktionsablauf, eine kurze Biografie zum Namensgeber der Reaktion, geläufige Vorgänge, Abwandlungen (wo vorhanden), Anwendungen, verwandte Reaktionen (wo vorhanden), Beispielexperimente, Querverweise. Mit mehreren Indizes (auch nach Reaktionstyp).












