Electronic Structure of Atoms
Chemistry for All
Versandkostenfrei!
Versandfertig in 1-2 Wochen
20,99 €
inkl. MwSt.

PAYBACK Punkte
10 °P sammeln!
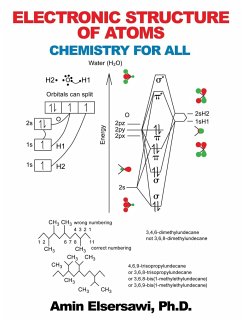
The book presents the quantum theory of the electronic structure of atoms and focuses on the electronic structures and reactivity of atoms and molecules. It shows how to draw molecules such as the oxygen and water to far more complex molecules, using molecular orbital theory, and hybridization of orbitals. It gives quite clear picture of molecular polarity, together with symmetrical and unsymmetrical distribution of an atom or molecule when developing a temporary (instantaneous) dipole. The book provides a clear and comprehensive summary of oxidative and reductive processes. Electronegativity ...
The book presents the quantum theory of the electronic structure of atoms and focuses on the electronic structures and reactivity of atoms and molecules. It shows how to draw molecules such as the oxygen and water to far more complex molecules, using molecular orbital theory, and hybridization of orbitals. It gives quite clear picture of molecular polarity, together with symmetrical and unsymmetrical distribution of an atom or molecule when developing a temporary (instantaneous) dipole. The book provides a clear and comprehensive summary of oxidative and reductive processes. Electronegativity on oxidation and reduction is also introduced. Examples are provided. It enables the reader to master the principles and applications of organic functional groups. Readers will find information quickly and easily about alkanes, alkenes, alkynes and arenes. Bonding with ¿ and ¿ is also introduced. It explains the fundamental principles of nomenclature methods, using IUPAC (International Union of Pure and Applied Chemistry) and enables the reader to apply it accurately and with confidence. The book is replete with examples for guidance and there are extensive and complicated figures to direct the reader to nomenclature quickly. It gives hands-on chemistry activities with real-life functions. It provides clear and thorough understanding of carbohydrates, polysaccharides, starch and glycogen, cellulose and chitin, nucleotide, nitrogenous hydroxyl and phosphate, lipids, protein, ester, lipoprotein, glycolipid, steroid, mucin, etc. it is a useful reference for health professionals, practicing physicists, chemists, and materials scientists.












