Lateral organization of cell-matrix proteins on the nanoscale
Versandkostenfrei!
Versandfertig in 6-10 Tagen
45,99 €
inkl. MwSt.

PAYBACK Punkte
23 °P sammeln!
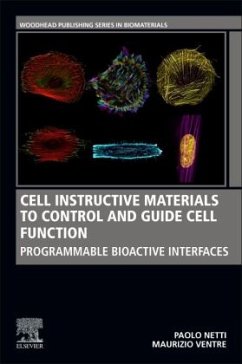
Cellular anchors are large accumulations of a multitude of multi-functional proteins and are known as focal adhesion sites. In this work, the spatial organization of the cell-matrix proteins Beta3-integrin, Talin, Kindlin 1&2, FAK, Paxillin, Vinculin, Zyxin, Alpha-actinin and Actin was analyzed, using the super-resolution microscopy technique PALM. It was demonstrated, that all cell-matrix proteins form distinct areas of varying densities inside single focal adhesions. In order to study the temporal alterations and formation of highly dense protein accumulations, the dynamic behavior of the ad...
Cellular anchors are large accumulations of a multitude of multi-functional proteins and are known as focal adhesion sites. In this work, the spatial organization of the cell-matrix proteins Beta3-integrin, Talin, Kindlin 1&2, FAK, Paxillin, Vinculin, Zyxin, Alpha-actinin and Actin was analyzed, using the super-resolution microscopy technique PALM. It was demonstrated, that all cell-matrix proteins form distinct areas of varying densities inside single focal adhesions. In order to study the temporal alterations and formation of highly dense protein accumulations, the dynamic behavior of the adhesion receptor Beta3-integrin was analyzed. It was shown, that force inhibition can induce structural rearrangements, also leading to the redistribution of the density inside adhesion sites. Dense domains could even have a particular signaling function, as it was demonstrated that the signaling protein FAK is primarily recruited to delimited areas inside focal adhesions, which could represent dense domains.