VIP PROTECTS TH2 CELLS BY DOWNREGULATING GRANZYME B EXPRESSION
Versandkostenfrei!
Versandfertig in 6-10 Tagen
39,99 €
inkl. MwSt.

PAYBACK Punkte
20 °P sammeln!
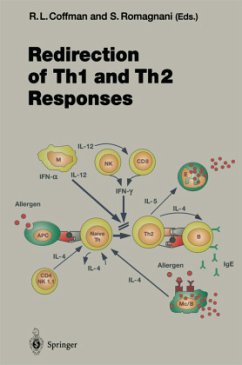
Following antigenic stimulation, CD4+ T cellsdifferentiate into Th1 and Th2 effectors, withdifferent cytokine profiles and differentphysiological functions. However, regardless of theinitial Th1/Th2 balance, the final immune responsedepends to a large degree on the regulation of Th1 orTh2 survival under specific conditions. The major purpose of this thesis was to identify andcharacterize the molecular mechanisms for theprotective effect of VIP on Th2 effectors in an invitro system. Our experiments revealed a surprisingnew mechanism involved in Th1/Th2 AICD, i.e. theinduction of enzymatically a...
Following antigenic stimulation, CD4+ T cells
differentiate into Th1 and Th2 effectors, with
different cytokine profiles and different
physiological functions. However, regardless of the
initial Th1/Th2 balance, the final immune response
depends to a large degree on the regulation of Th1 or
Th2 survival under specific conditions.
The major purpose of this thesis was to identify and
characterize the molecular mechanisms for the
protective effect of VIP on Th2 effectors in an in
vitro system. Our experiments revealed a surprising
new mechanism involved in Th1/Th2 AICD, i.e. the
induction of enzymatically active granzyme B (GrB)
upon T cell receptor restimulation. In wild-type Th1
and Th2 cells, apoptosis is mediated through both Fas
signaling and GrB induction. In lpr (Fas mutant) Th1
and Th2 effectors T cell receptor restimulation
results in apoptosis mediated solely by GrB,
suggesting that GrB induction is independent of Fas
signaling. VIP induces the survival of wild-type Th2
effectors by preventing the induction of GrB and the
upregulation of FasL expression in Th2, but not Th1
cells.
differentiate into Th1 and Th2 effectors, with
different cytokine profiles and different
physiological functions. However, regardless of the
initial Th1/Th2 balance, the final immune response
depends to a large degree on the regulation of Th1 or
Th2 survival under specific conditions.
The major purpose of this thesis was to identify and
characterize the molecular mechanisms for the
protective effect of VIP on Th2 effectors in an in
vitro system. Our experiments revealed a surprising
new mechanism involved in Th1/Th2 AICD, i.e. the
induction of enzymatically active granzyme B (GrB)
upon T cell receptor restimulation. In wild-type Th1
and Th2 cells, apoptosis is mediated through both Fas
signaling and GrB induction. In lpr (Fas mutant) Th1
and Th2 effectors T cell receptor restimulation
results in apoptosis mediated solely by GrB,
suggesting that GrB induction is independent of Fas
signaling. VIP induces the survival of wild-type Th2
effectors by preventing the induction of GrB and the
upregulation of FasL expression in Th2, but not Th1
cells.