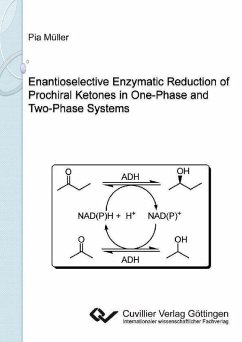
A strategy for the enantioselective synthesis of chiral short
chain alcohols using biocatalysis is developed employing
substrate and enzyme dependent in situ cofactor
regeneration. Enzyme characterization shows strong
dependence of activity on diverse reaction parameters. The
obtained results are transferred to one-phase batch synthesis
of (R)- and (S)-2-butanol where conversion and
enantioselectivity are strongly dependend on reaction
conditions. Due to limitations like low substrate solubility the
synthesis is transferred to two-phase reaction systems where
MTBE is the solvent of choice. Conversion and selectivity are
positively influenced. The same is found for two-phase
reactions in a continuous reaction set-up. With the optimum
reaction conditions obtained from the batch experiments
conversion and selectivity are improved. ADH and cofactor
show exceptionally high stability and high TTN. Together with
further development of a work-up strategy the continuous
two-phase reaction set-up will be a strong tool to produce
enantiopure short chain alcohols on preparative relevant
scale.
Dieser Download kann aus rechtlichen Gründen nur mit Rechnungsadresse in A, B, BG, CY, CZ, D, DK, EW, E, FIN, F, GR, HR, H, IRL, I, LT, L, LR, M, NL, PL, P, R, S, SLO, SK ausgeliefert werden.